Part:BBa_K731201
Arabinose inducible araC-pBAD promoter
This E. coli promoter is positively regulated by arabinose.
araC is the regulator protein: in absence of arabinose the protein binds to araI1 and araO2 and the DNA forms a loop that prevents RNA polymerase from binding the promoter. When arabinose is added to the culture it binds to araC, disabling the interaction with araI1 and araO2 sites, and also allowing it to bind araI site, promoting transcription.
Promoter activity is also influenced by glucose levels: in its absence cAMP levels are high and the the complex cAMP+CAP (catabolite activator protein) binds to lacI promoting transcription. In presence of glucose, cAMP levels are low and promoter activity is inhibited.
This part differs in several point mutations from part BBa_I0500, mainly in araC coding sequence.
Usage and Biology
This part has been successfully operated both in pSB1C3 and the low copy vector pSB3C5, in which it was characterized.
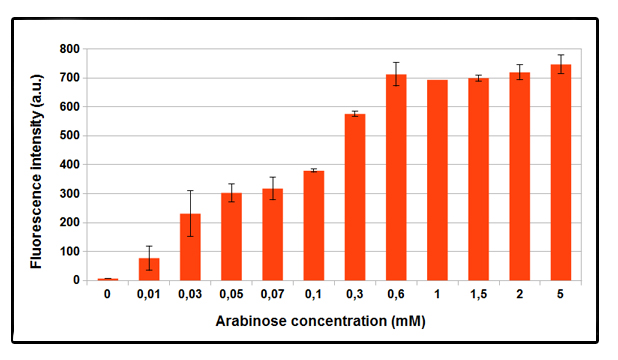
FIGURE 1. GFP expression with different concentrations of arabinose, after 4 hours of induction.
NEB10beta cells transformed with part BBa_K731250 (in pSB3C5) were grown in LB broth and induced at OD_600 = 0.6 . Higher GFP expression was observed as the arabinose concentration increases. No difference in fluorescence intensity between not induced and control NEB10beta cells was observed. Data shown is a mean of three different measurements.
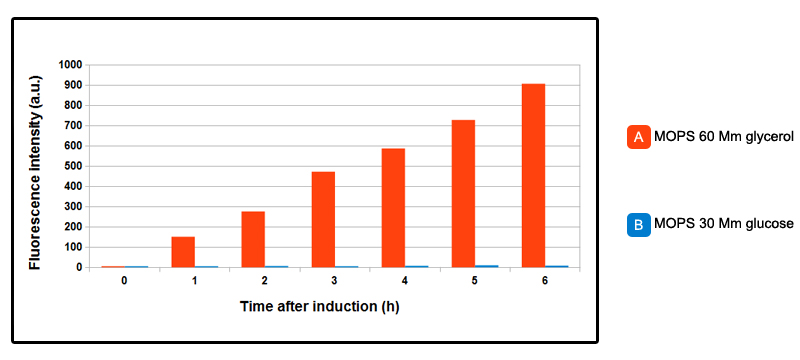
FIGURE 2. GFP expression over time, after induction.
NEB10beta cells, transformed with part BBa_K731250 (in pSB3C5) were grown in LB broth until OD_600= 0.3 then centrifuged and resuspended in equal amount of minimal MOPS medium and induced at OD_600 = 0.6 with 5mM arabinose concentration. MOPS A is 60mM glycerol as carbon source, MOPS B is 30mM glucose: in the chart, data relative to MOPS A are shown in orange, the ones relative MOPS B are in blue. In absence of glucose, increasing linear expression over time of GFP is observed, while in MOPS B, in presence of glucose, promoter is inhibited, and fluorescence levels are comparable with control NEB10beta cells. In a later experiment done with a superfolderGFP-tagged CysE enzyme, some fluorescence in presence of glucose was observed. You can see the results on this page.
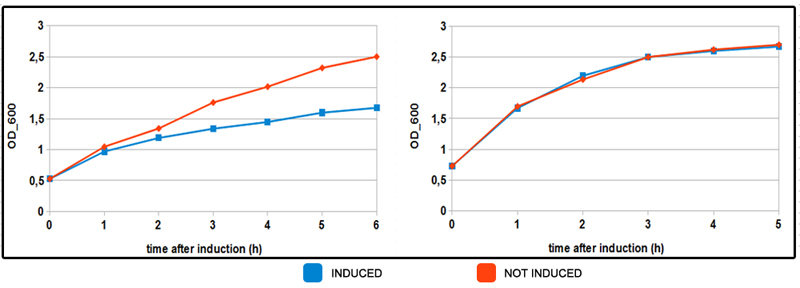
FIGURE 3. Induced and not induced culture growth in MOPS mediums.
NEB10beta cells, tranformed with part BBa_K731250 (in pSB3C5) were grown in LB broth until OD_600= 0.3 then centrifuged and resuspended in equal amount of minimal MOPS medium and induced at OD_600 = 0.6 with 5mM arabinose concentration. On the left OD mesurements of coltures growing in minimal MOPS A medium (glycerol, 60mM). In orange not induced culture, in blue induced (arabinose 5mM). Same colors are used on the chart on the right for induced-not induced bacteria with MOPS B medium (30 mM glucose). Inhibition of growth is observed in MOPS A when induced. There seems to be no effect in MOPS B. This particular behaviour is probably due to energy assessment while growing on glycerol as the sole carbon source. More energy is invested in protein production as observed in FIGURE 2.
Effect of glucose on arabinose-induced expression
Group: (Team iGEM CMUQ 2019)
Author: CMUQ iGEM Bio Team
Summary: The below experiments provide additional data about the araC promoter described on this page; specifically, the effect of glucose on the activity level of the promoter.
We investigated the effect of glucose on the levels of expression of araC controlled GFP by measuring fluorescence levels. The strain of E. coli used was E. coli BL21 (DE3). The bacteria were transformed with pLysSBL-21 (DE3) from Promega (catalog number L1198 ) plasmid, to which we integrated the inducible araC-pBad promoter with GFPmut3b, a strong ribosome binding site (Bba_K731250).
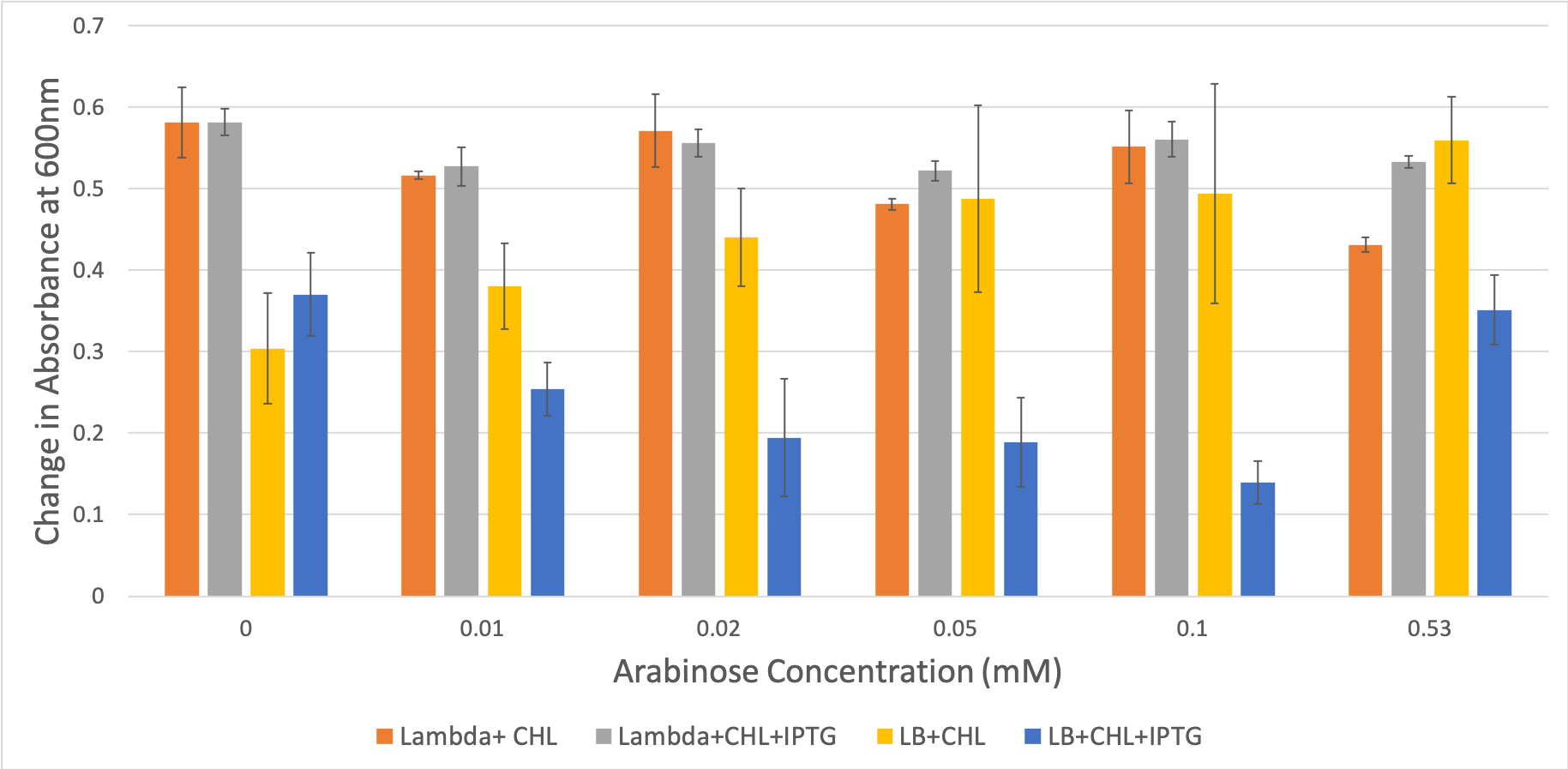
FIGURE 4. Concentration of E. coli cells across various conditions.
The concentration of bacteria remained relatively constant across the different concentrations of arabinose. Hence, arabinose does not affect the growth rate of cells and changes in levels of GFP shown in Figure 5 may be attributable to promoter activity. However, we note that LB+chl+IPTG had a slower growth compared to the other permutation of the media.
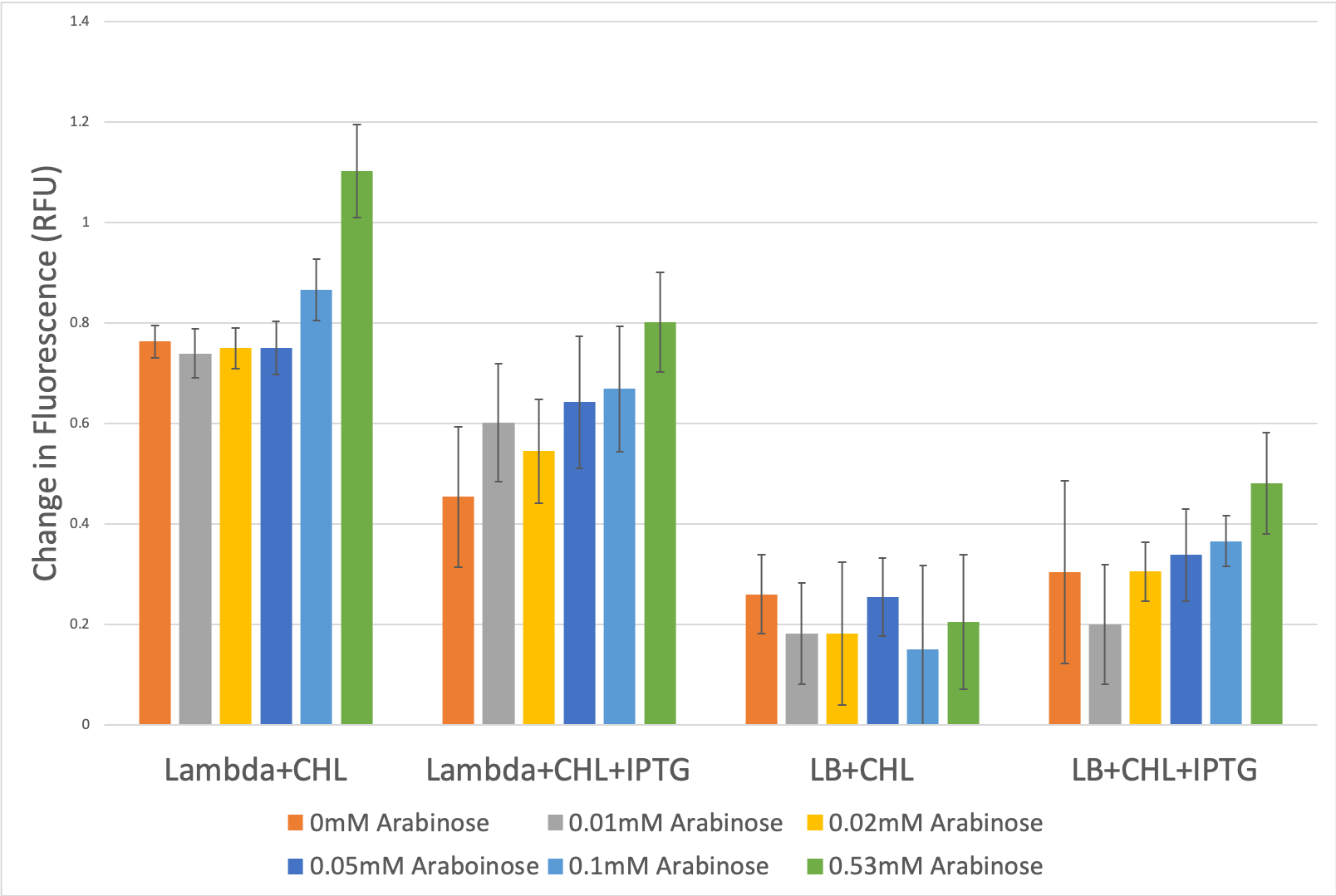
FIGURE 5. Expression levels of GFP agains E. coli media composition
Expression levels of GFP in various different conditions of E. coli environment was measured. We observe that an increase in arabinose levels leads to an increase in the levels of GFP expressed. We also observe that the presence of glucose in the media significantly reduces the levels of GFP expressed. Thus we conclude that araC promoter activity is positively regulated by arabinose but negatively regulated by glucose.
Characterization of araC-pBAD promoter
Group: (Team iGEM Tongji_China 2020)
We transferred BBa_K731250 into Escherichia coli DH5α and induced with different concentrations of arabinose. The corresponding relative fluorescence intensity was observed to verify whether the promoter could be properly induced to express.
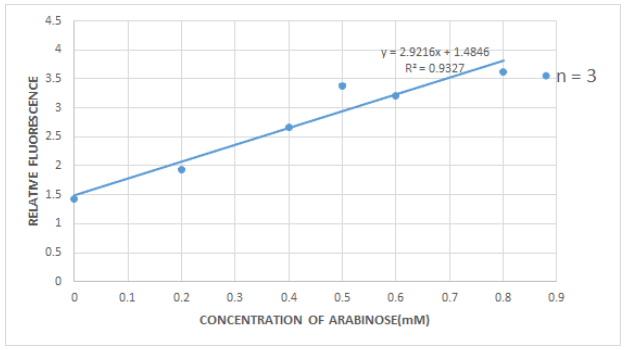
FIGURE 9. Relative flourescence intensity of E.coli-K731250 induced by different concentration of arabinose.
Measured by excitation wavelength of 475 nm, emission wavelength of 515 nm. Relative fluorescence is calculated by the formula below, LB medium as blank. The result was similar to the characterization by the CMUQ iGEM Bio Team.
FIGURE 10. Calculating formula.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 1144
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 979
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI site found at 961
//direction/forward
//chassis/prokaryote/ecoli
//promoter
//regulation/positive
//classic/regulatory/other
| None |